
Or their B values, we would see something really interesting, and that's that all of them have a volume of zero at the exact same temperature which is negative 273.15 degrees Celsius which is also zero Kelvin. If we extrapolate these values out to find their Y intercepts, So that's kind of why this straight line stopped, but we can extrapolate this line all the way down as well.

With water vapor, the boiling point is 100 degrees Celsius With methane, the boiling point would be about negative 100 degrees Celsius, but we could kind ofĮxtrapolate this line down. That's because that all of these gases turn into liquid atĭifferent temperatures. You can also see that the lines are coming to a stopping The slopes are different, it's because the different gas samples in this example would have different number of moles.
CHARLES LAW PLUS
Our X is our temperature, so if we fill that all the way in here, we'd have V is equal to MT plus B. Values that we're using in this graph, our Y is our volume so we would see that Y is equal to V. In Y intercept form, that would look like Y equals MX plus B. But all of these gases can be plotted in a straight line. This blue line might indicate water vapor, water gas, steam, and this yellow line would indicate hydrogen gas. As we increase the temperature, we're increasing proportionally the volume that the methane's taking up. Then we've got this green gas, and this might be methane, and we're seeing the same thing. This straight line is showing this down to at zero degrees Celsius, we've got just a little over three liters that this helium is taking up. As we decrease this temperature, the volume is going toĭecrease proportionally. Is taking up a volume of about 5 liters right here. So at about 300 degrees Celsius, this helium we can see This is what a plot of volume expansion would look like for four different gases as we're increasing the temperature. I think I can show thisĪ little bit more clearly if I use a plot of gases Or the volume increases proportionally to the In fact, the volume increases directly with the temperature, As you heat a system of gas, the volume will also increase. This is what the piston would look like after the heat was applied. We still have six green particles of gas. Was taking up more volume even though there's the same If I show this same piston after the heat was applied, we'd see that the gas

That the volume of the gas will also increase. With the same amount of particles as you heat this piston, so let me apply some heat here, and what we'll see is Under constant pressure because as the atmosphere is pushing down on top of the piston, then the pressure of the gas pushing up is going to equal the atmosphere. With gas and temperature, he found that if you heat a gas in a closed container say like a piston, so I've got a piston here, and I'll fill it with gas. He was the first person to fill up a hot airīalloon with hydrogen gas and fly solo. This French physicist also liked experimenting with gas, and it actually turns out If I didn't look up the pronunciation for this man's name, I probably would have said Jacks Charles, but it's Jacques Charles.
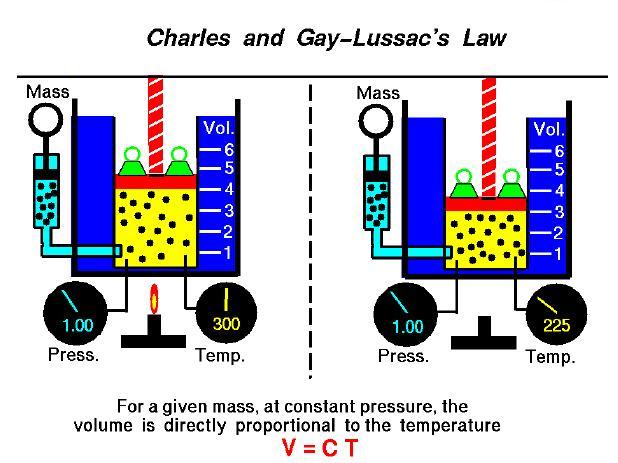
About 100 years after Robert Boyle, there came a French physicist Now I want to talk about the experiment that led to the V equals Talking about Boyle's law, and the experiments that led to the PV part of the ideal gas equation.


 0 kommentar(er)
0 kommentar(er)
